Celiac disease and intestinal damage
Celiac disease is an autoimmune disorder with genetic predisposition , which causes villous atrophy due to gluten exposure.
Currently the only treatment available is to follow a strict lifelong gluten-free diet (GFD) This follow up achieves to restore the function and normal aspect of the intestinal villi and, thus, the correct nutrient absorption.
How does gluten cause damage to the small intestine?
Gliadins and glutenins are components of gluten, and are also present in wheat, oats and barley. In gastrointestinal digestion, these proteins are not completely degraded, but smaller degradation fragments remain and pass to the intestine, they are called gluten immunogenic peptides (GIP). These GIPs are the cause of the pathological lesion in the intestine of celiac patients.
Continued exposures to low amounts of gluten cause inflammation, sometimes subclinical, which can increase the risk of long-term complications, including lymphoma, osteoporosis, anemia, malnutrition (1,2). In non-celiac people, GIP do not cause any damage.
Determination of GIP to demonstrate the efficacy of a promising pharmacological therapy for celiac disease
Following a lifelong gluten-free diet without transgressions is very difficult and often leads to nutritional deficiencies. Hence, other therapeutic options are sought. In a phase 2b clinical trial, led by Dr. Murray and Dr. Syage, a “gluten challenge” was incorporated to test the efficacy of a drug, latiglutenase, a protease capable of efficiently degrading GIP in the laboratory.
In this study, celiac patients received 2 g of gluten per day (the equivalent of a slice of bread) for a period of 6 weeks. One group received latiglutenase vs the other, which received placebo. Before and after the period of exposure to gluten, the symptoms were noted and different parameters were measured to check the status of the intestinal mucosa of the patients, that is, whether or not there was intestinal damage: histological analysis, measuring the relationship between height of the villi and the depth of the crypts (Vh:Cd); the density of intraepithelial lymphocytes; and serological tests for antibodies that is elevated due to gluten consumption.
Weekly urine samples were also collected from all patients before, during, and after the gluten exposure period. They were analyzed for the presence of gluten, specifically the presence of GIP. For this, the IVYCHECK GIP Urine immunochromatographic assay was used with IVYCHECK Reader, capable of measuring the concentration of GIPs present in each urine sample.
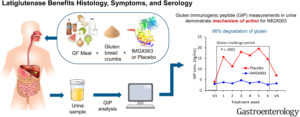
Figure 1. Degradation of GIP by the action of latiglutenase during the 6-week “gluten-challenge” compared to subjects who received placebo. Obtained from Murray et al., 2022.
One of the findings of this study was the great action capacity of latiglutenase in the degradation of gluten and its peptides resistant to gastrointestinal digestion. 95% of the gluten ingested by the patients was degraded by latiglutenase in the stomach.
This is also a relevant and significant finding, since it is demonstrated for the first time in a “gluten challenge” (voluntary and controlled exposure to gluten), that the presence of GIP correlates with intestinal damage, as observed in patients from the study that ingested placebo instead of the drug; in other words, those patients who ingested latiglutenase had no detectable traces of GIP in the urine, while those who ingested placebo had traces in the urine and greater villous atrophy (3,4).
Promising therapeutic research for celiac patients and tools to measure their efficacy– GIP and intestinal damage– gastrojournal.org/article/S0016-5085(22)00901-5/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F.
Bibliography:
- Syage JA, Green PHR, Khosla C, Adelman DC, Sealey-Voyksner JA, Murray JA. Latiglutenase Treatment for Celiac Disease: Symptom and Quality of Life Improvement for Seropositive Patients on a Gluten-Free Diet. GastroHep. 2019;1(6):293-301. doi:10.1002/ygh2.371
- Kelly CP, Bai, JC, Liu E, Leffler DA. Celiac disease: clinical spectrum and management. Gastroenterol 2015;148:1175-1186.
- Green PHR, Lebwohl B, Greywoode R. Celiac disease. J Allergy Clin Immunol 2015;135:1099-1106.
- Murray JA, Syage JA, Wu TT, Dickason MA, Ramos AG, Van Dyke C, Horwath I, Lavin PT, Mäki M, Hujoel I, Papadakis KA, Bledsoe AC, Khosla C, Sealey-Voyksner JA, & CeliacShield Study Group (2022). Latiglutenase Protects the Mucosa and Attenuates Symptom Severity in Patients With Celiac Disease Exposed to a Gluten Challenge. Gastroenterol 2022; S0016-5085(22)00901-5.

